New
New
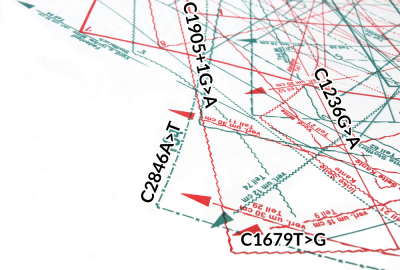
Human Cell Line Authentication on LabCard
LabCards are intended for simple transport of a cell sample or genomic DNA of human cell lines. Samples applied to the LabCard do not need to be transported chilled. STR analysis is performed as part of the service.